Lab: Dependency of Boiling Points on IMFs
The purpose of this lab was to determine the boiling range of different molecules and observe how the strength of their IMFs affect the temperature change involved with evaporation of the compounds.
Graph for Methanol and Propanol
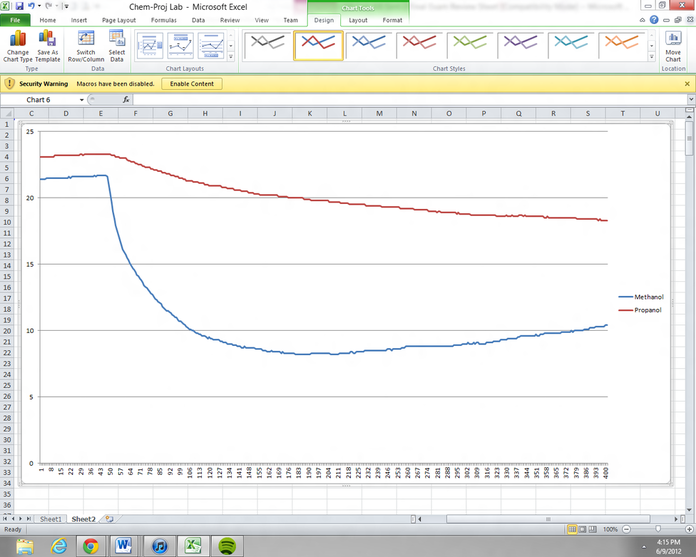
Shown above is the data obtained from the two temperature probes with swabs of filter paper wrapped around them. One was dipped into a Methanol solution and the other a Propanol solution. As it had been predicted, the smaller molecule between equal degrees of polarity would evaporate with a greater temperature change i.e. methanol. The graph of time versus temperature for Methanol is indicated with a blue line and with a red line for Propanol.
Videos with Instructions
|
|
|
Data Table, Analysis, and Questions
| apchem_imf_dataanalysisquestions.docx | |
| File Size: | 29 kb |
| File Type: | docx |
